Introduction: Based on the efficacy of the fixed-duration venetoclax and rituximab combination (VenR) in the setting of relapsed/refractory (R/R) patients with chronic lymphocytic leukemia (CLL), we investigated the efficacy and safety of the front-line VenR regimen in young (≤65 years) and fit patients with CLL and an unfavorable biologic profile in the GIMEMA LLC1518 VERITAS trial. Here, we report the 36-month updated results of this phase II, single-arm, multicenter, front-line study for young patients with CLL carrying an unmutated IGHV profile and/or a TP53 disruption.
Methods: Seventy-five CLL patients requiring treatment, according to the International Workshop on CLL (iwCLL) criteria, were enrolled between October 2018 and May 2020. Treatment consisted of the five-week Ven ramp-up, six-monthly courses of the VenR combination, followed by six monthly courses of Ven single agent. Patients received tumor lysis syndrome (TLS) prophylaxis with urate-reducing agents and oral or iv hydration. The primary endpoint of this study was the complete remission (CR) rate assessed at the end of treatment (EOT, month 15) according to the iwCLL criteria. Measurable/minimal residual disease (MRD) was assessed on the peripheral blood (PB) and bone marrow (BM) by ASO-PCR at baseline, EOT and during the follow-up. MRD was categorized as undetectable (uMRD) with a cut-off of <1 cell in 10,000 leukocytes.
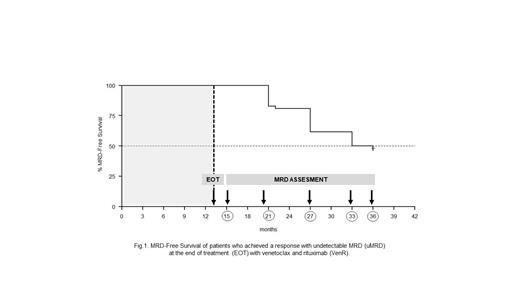
Results: The median age of patients was 54 years (range 38-65); 96% had unmutated IGHV, 9 (12%) had TP53 disruption, and 4% showed mutated IGHV with TP53 disruption. At the EOT, the overall response rate (ORR) was 94.7%, with a CR rate of 76%. On an intention-to-treat basis, 52 (69.3%) patients showed a response with uMRD in the PB and 44 (58.7%) in the BM. At 33 months from the EOT, uMRD was maintained by 50% of patients who had achieved an uMRD in the PB and by 57% of those with a prior uMRD in the BM. The 33-month PB MRD-free survival was 50% (Figure 1). In multivariate analysis, factors associated with a significantly shorter MRD-free survival were the presence of an unmutated IGHV associated with a TP53 disruption (HR: 3.43 [95%CI: 1.13-10.4]; p=0.029), Binet B stage (HR: 0.16 [95%CI: 0.06-0.43]; <0.001) or, Binet C stage (HR: 0.19 [95%CI: 0.07-0.52]; p=0.001), while the clinical response, CR, or PR, did not show a significant impact. Three patients have died due to COVID-19. Despite iv hydratation, the administration of anti-uric acids, fatal TLS occurred in a patient who used self-administered fentanyl patches for analgesis purposes during the ramp-up phase. The 36-month OS was 96%. A further lymphoproliferative disorder was diagnosed in 2 patients (follicular lymphoma, 1; large granular cell leukemia, 1) and a second malignancy in 1 (prostate cancer), while no cases of Richter transformation were recorded. No unexpected adverse events were observed during the follow-up.
Conclusions: The updated data from the VERITAS trial show that in young and fit patients with adverse genetic characteristics the front-line VenR combination was an effective regimen with high rates of deep responses with uMRD. Furthermore, at 33 months from the EOT, uMRD was maintained in the PB and BM in about half of the patients.
Disclosures
Mauro:Abbvie, Janssen, Beigene, Astra Zeneca, Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Reda:Abbvie, Janssen, Beigene, Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: At the time of the presented study Dr Reda was employed as MD at the 3Hematology Department, Foundation IRCCS Ca' Granda Ospedale Maggiore Policlinico of Milan. Currently Dr Reda is employed at ASTRA-Zeneca , Speakers Bureau. Sportoletti:Abbvie, Janssen, Beigene, Astra Zeneca, Takeda, Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Gaidano:Abbvie and Janssen: Speakers Bureau; Abbvie, Astra-Zeneca, BeiGene, Incyte, Janssen, Lilly: Other: Advisory board. Marasca:Abbvie, Janssen, Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Murru:Abbvie, Janssen, Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Rigolin:Janssen, Abbvie, Gilead, Astra-Zeneca, Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Scarfo:AbbVie: Consultancy; BeiGene: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; Lilly: Consultancy; Octapharma: Speakers Bureau. Tani:Abbvie, Jansen-Cilag, Incyte: Membership on an entity's Board of Directors or advisory committees. Arcari:Janssen, Abbvie, Takeda, Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Musuraca:Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Pepe:Abbvie, Astra-Zeneca, Beigene: Membership on an entity's Board of Directors or advisory committees, Other: travel grant. Visentin:Janssen, Abbvie, Astra-Zeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Vitale:Janssen, Abbvie, Astra-Zeneca, Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Del Giudice:Astra-zeneca, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Vignetti:ER Congressi: Honoraria; Dephaforum: Honoraria; Uvet: Honoraria; AbbVie: Honoraria; Novartis: Speakers Bureau; IQVIA: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal